Real Assets
We build future energy systems and resilient infrastructure, backing emerging opportunities in technology, land and water.
Real Assets
Private Equity & Ventures
Real Assets
Private Equity & Ventures
Claire Jervis explores the role of AI in healthcare and medical device R&D and innovation - including within our portfolio - with a word of warning on the risks posed.

Gates shrugged.
Nor was he impressed by the company’s documentary, ‘Artificial Gamer’, which showed an OpenAI agent defeating the World Champions of DOTA 2, a strategy-based video game.1
Gates saw AI’s potential as a tool to support PhD-level research – not for playing childhood toys. The team finally won him over with a demonstration of GPT-2, which could just about summarise documents and answer questions.
Microsoft invested $1 billion in OpenAI on the potential they saw in the GPT model – and so began the relentless march to harness the potential of AI in advanced research.2
Six years later, GPT-5 is lightyears ahead of the model presented to Gates. And in 2024, it crossed a seminal milestone – reaching human ability in solving PhD-level science questions.
This could have massive implications across many areas of the economy. But one area which is especially ripe for AI-driven gains in advanced research is Healthcare.
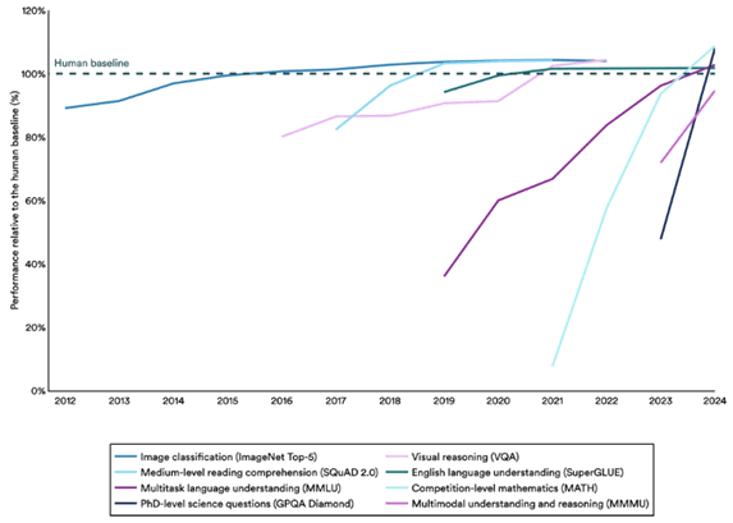
Figure 1: Select AI Index technical performance benchmarks vs. human performance.3
R&D costs for pharmaceutical pipelines have been steadily rising over the last 10 years, and productivity has been falling.4
This is famously captured by ‘Eroom’s Law’, the observation that the number of new molecules approved by the FDA per $bn in R&D spending has been in steady decline since the 1950s.
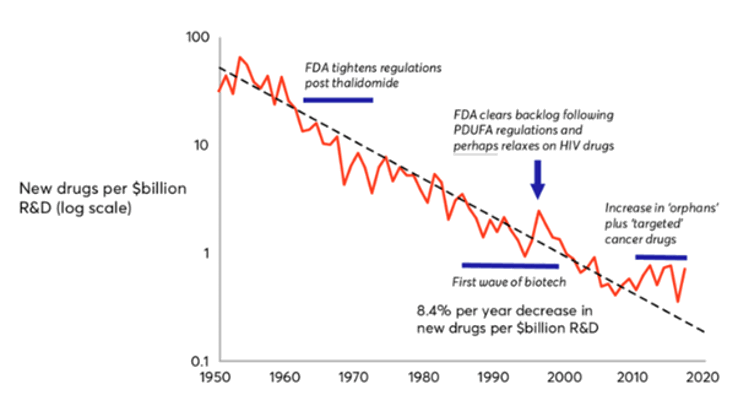
Figure 2: Eroom’s Law: the number of new molecules approved by the FDA per $bn global R&D spending.5
There is evidence that pharma companies are already beginning to adopt AI to solve this problem. The use cases we hear companies talk about the most relate to improvement of clinical trials – AI can enable better patient selection, trial design, and predictive modelling to reduce failures.
Meanwhile, a meta-study from this year finds evidence of big pharma using AI to speed up lead molecule and target identification, and using biodata to improve drugs’ safety profiles.6
However, due to the long lead times on new drug development (typically 10-15 years), and strict regulation, it may take time for us to see real evidence of this improving pharma innovation and R&D productivity.
Medical devices, or MedTech, are not burdened by the same wicked R&D environment as their peers in the pharma industry.
A new medical device only takes around 3-7 years to reach approval in the US.
The regulatory standard is also much lower – most medical devices can be approved on the basis of showing ‘substantive equivalence’ to another device on the market. When evaluating new devices, clinical trials can be considered a ‘nice to have’.7
Applying AI to the development of medical devices therefore won’t be as transformative for improving R&D productivity compared to pharma.
But the lower regulatory burden means we can already see evidence of AI-augmented medical devices entering patient care regimes, and adoption of this technology could still have the potential to transform patient outcomes.
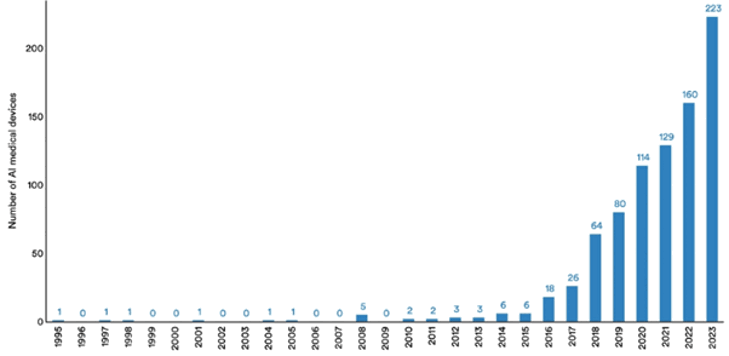
Figure 3: Number of AI medical devices approved by the FDA, 1995-20238
Siemens Healthineers is already using AI to improve cancer treatment
We are already seeing evidence of this in our portfolio.
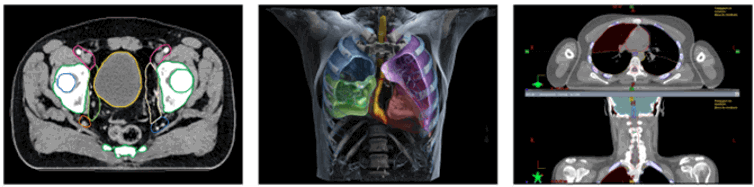
Siemens Healthineers is the global leader in imaging technology (like PET and CT scanners) and radiation oncology machines. They are at the forefront of leveraging AI to support early diagnosis, and better treatment, of diseases including cancer.
The FDA lists a raft of AI technologies, created by Siemens Healthineers, already approved for patient care.9 One solution uses AI to provide automatic contouring of organs at risk of cancer. This has historically been a major bottleneck in radiation therapy planning as it is time-consuming for doctors, and errors are considered a high-risk part of the radiotherapy process.10
This is just one example of how MedTech companies are using AI to improve patient outcomes. We expect this will become increasingly prominent in patient care regimes, and as a competitive differentiator for companies.
While AI has the power to supercharge health care innovation and patient care, this is not without risk.
Several studies have found that AI tools used by doctors may lead to poorer outcomes for women and ethnic minorities. AI tools have been seen to downplay the severity of female patients’ symptoms. Large language models (LLMs) were said to display ‘less empathy’ towards Black and Asian patients.11
This is largely down to the fact that LLMs are trained on data that reflects pre-existing biases.
There is empirical evidence that women and ethnic minorities are consistently underrepresented in clinical trials.12
While the problems highlighted by the studies above are harder to measure (downplaying severity, not displaying empathy), it is easy to imagine how gender and racial biases may have crept into AI training data. On gender, Naga Munchetty has compiled a tome of anecdotal evidence supporting the claim that doctors may not take female patients seriously.13
It is the responsibility of health care companies and physicians to be aware of the biases that may exist in these tools – and the responsibility of tech companies to negate them.
Most importantly, it is the responsibility of those in positions of power to safeguard objectivity in health care, and prevent biases and misinformation from entering training data in the first place.14

Claire Jervis
Associate Fund Manager
Foresight Capital Management
For further information, please get in touch with your regular Foresight contact or the client team on the details below:
2 A brilliant account of OpenAI’s history can be found in Empire of AI, by Karen Hao of the MIT Technology Review.
3 Stanford AI Index, https://hai.stanford.edu/ai-index/2025-ai-index-report
Foresight Group LLP does not offer legal, tax, financial or investment advice and the information on this website should not be construed as such. We recommend investors seek advice from a regulated financial adviser. The opportunity described in this document may not be suitable for all investors. Any such investment decision should be made only on the basis of the Fund scheme documents and appropriate professional advice.
Foresight Group LLP acts as investment manager and is authorised and regulated by the Financial Conduct Authority with Firm Reference Number 198020 and has its registered office at The Shard, 32 London Bridge Street, London SE1 9SG.
OEICs
An investment in FP Sustainable Future Themes Fund, FP Foresight Global Real Infrastructure Fund, FP Sustainable Real Estate Securities Fund, FP UK Infrastructure Income Fund or FP WHEB Sustainability Impact Fund and Liontrust Diversified Real Assets Fund (together the “Funds”) should be considered a long-term investment that may be higher risk. Portfolio holdings are subject to change without notice.
The Authorised Corporate Directors FundRock Partners Limited (registered office at Hamilton Centre, Rodney Way, Chelmsford, England, CM1 3BY) and Liontrust Investment Partners LLP (registered office 2 Savoy Court, London WC2R 0EZ), are authorised and regulated by the Financial Conduct Authority with Firm Reference Numbers 469278 and 518552 respectively. The Funds are incorporated in England and Wales.
ICAVs
An investment in the WHEB Sustainable Impact Fund and the WHEB Environmental Impact Fund (together the “Funds”) should be considered a longer-term investment that may be higher risk. Portfolio holdings are subject to change without notice.
The Manager of the Funds is FundRock Management Company S.A., authorised and regulated by the Luxembourg regulator to act as UCITS management company and has its registered office at Airport Center Building, 5, Heienhaff, L-1736 Senningerberg, Luxembourg.
We respect your privacy and are committed to protecting your personal data. If you would like to find out more about the measures, we take in processing your personal information, please refer to our privacy policy, which can be found at http://www.foresight.group/privacy-policy.